Let’s talk about alcohol. This information is for those who need to change the strength of the alcohol/water solution, let say for good infusion or liquor homemade drink. If you are a professional chemist and you’ll find any errors from my side, please let me know. The popular sources are usually wrong or incomplete, so I had to figure out some stuff on the fly.
How correctly dilute the pure ethanol to a standard 40% ABV or to any possible strength?
It seems easy and it is, if you know the main trick. To save your time, here is the general rule. To make 40% ABV from alcohol: take 3 parts of water (aka 600ml) and add 2 parts of alcohol (aka 400ml ). You are done. You can stop reading here and go to make 40% ABV with very good precision. Do not forget to like my post before you go 🙂
Wait a second, if you remember some chemistry, you may ask: the alcohol that you have is not a pure 100% ethanol(say 95%), and what about the effect of volume contraction? You are right, but in this case the impurity of alcohol and the contraction effect are canceling each other. The actual EtOH concentration in this rule would be 39.07% ABV. Not bad for simple solution. In order to confirm this number you may use a calculator “Dilute” I’ve programmed.
Before going to describe the accurate calculations and procedures, I need to make one thing clear (It is the main cause of confusion among people). What is ABV?
ABV is the concentration of alcohol by volume. Or “It is defined as the number of millilitres (mL) of pure ethanol present in 100 mL of solution at 20 °C” (wiki).
Two main points here:
It is a “volume” of EtOH (not weight)
It is in “solution”. Here We have a case that 2+2 does not equal 4. Indeed, the total Volume of solution is less than the sum of Ethanol and Water Volumes.
Why this weird definition was accepted by the international community? It was accepted a long time ago because measurements of volumes was easy to make rather than weighting.
A note for American readers. Traditional American unit to measure the alcohol strength is “proof”. it happens to be twice the ABV. (aka 80 Proof = 40% ABV). The origination of this unit is going from the XVI century, when gunpowder was used as “proof” that drink has enough alcohol in it. The powder was soaked in alcohol and can burn only if the alcohol concentration was not less than 57% ABV. That was considered as a 100% proof of a decent drink. In England they used sweet Rum for this purpose. Either Americans used a cleaner alcohol or just changed the scale the 100% proof in US became 50% ABV.
A note for Russian reader. A Russian traditional unit for vodka was 40% by weight (or ABW). It was started around 1890th and was continued up to around 1970th. When market became more open for foreign drinks (measured in ABV) the Russian industry standard switch to ABV also. The vodka become “40% об”. This ob means volume(объем не обороты:). Note, that 40%ABV is equal to 33.8% ABW. That means that Soviet people were deprived of 6% of alcohol from the day of the conversion.
One more notice, before we are going into procedures: The standard ethanol available at the liquor shop is 95% ABV. Why not 100%? The distillation process cannot get more purity. The pure alcohol evaporates before the water and it is collected in the distillation cube. That is the nature of distillation. However, at 95% ABV mixture, the water and ethanol are vaporized with the same speed. That’s why, at this concentration there is no way to separate Water and Alcohol by distillation. Other chemical methods can be used to dehydrate alcohol, but they are expensive and do not make economical sense in drinking alcohol production.
In infusion preparations we may have two specific problems:
A. How much water we should add to 95% alcohol in order to obtain the mixture of predefined concentration (aka 40% ABV)?
B. If We have an infusion(like cranberry) that drops its strength during the production. We want to fortify the strength back to predefined concentration. How much 95%EtOH we should add to fortify our drink?
In general, we want to know what would be the concentration of product if we mixing Alcohol liquid (Volume: V1, ABV: A1) with another alcohol liquid (Volume: V2,ABV: A2).
Here is the link to math details: https://sergeyand.s3.amazonaws.com/AlcoMath.html
Having deduced all needed equations we can program calculators for the practical problems.
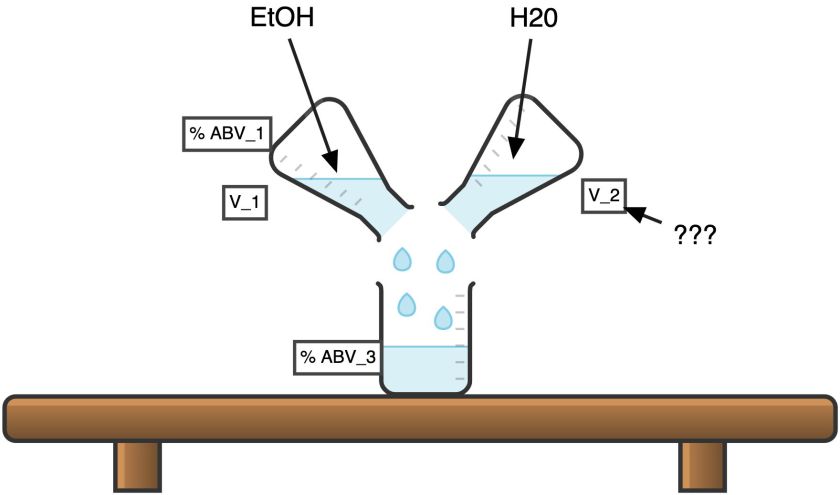
Question A: “Dilute Calculator”
Given Alcohol of high strength ABV_1 in volume V_1. How much water we should add in order to obtain the mixture of given strength ABV_3?
Question B: “Fortify Calculator”
Given diluted alcohol/water mixture ( ABV_1, V_1) you want to change it concentration by adding another alcohol mixture(Fortifier). You want to reach a predetermined concentration of the product. What volume of fortifier you should use.

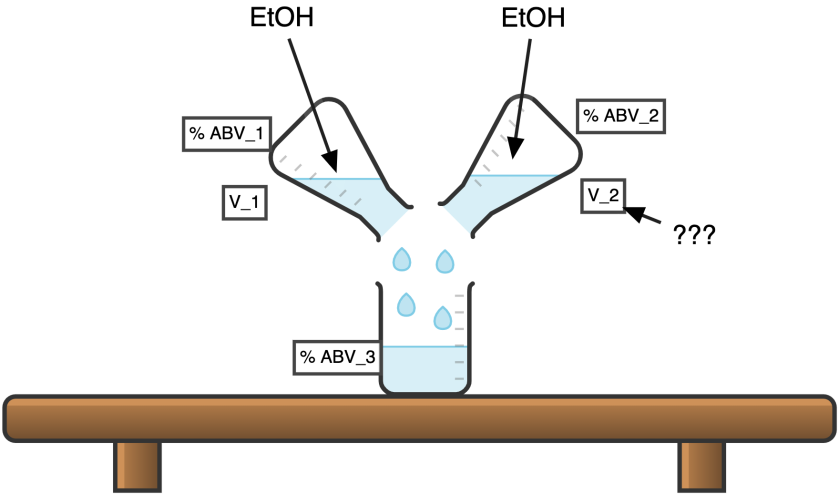
And Finally “Mix Calculator”,
Question C:
You mixing two different solutions of the alcohol (V1,ABV1) and (V2,ABV2)
What would be the resulting solution (V3,ABV3)?
Combining calculations above, experimental data and javascript coding we’ve got a good calculators to solve most of infusion, extraction and dilution problems.